

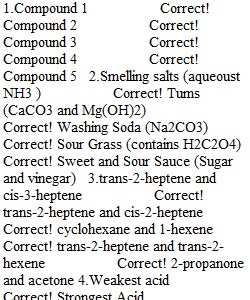
Q Question 1 0.5 / 0.5 pts Match the functional groups in these molecules (compounds A-E) to the appropriate classification. Question 2 0.5 / 0.5 pts Identify the following substances as acidic, basic, or neutral. Question 3 0.5 / 0.5 pts Determine the relationship between the following pairs of compounds. Question 4 0.5 / 0.5 pts Match the description to the acid (A-E). Name Acid pKa A. oxalic acid H2C2O4 1.19 B. citric acid H3C6H5O7 3.08 C. acetic acid HC2H3O2 4.74 D. carbonic acid H2CO3 6.37 E. boric acid H3BO3 9.24 Question 5 0.3 / 0.5 pts Match the situation or pH to the [H+]. A) [H+] = 1 x 10-12 B) [H+] = 1 x 10-8.4 C) [H+] = 1 x 10-7 D) [H+] = 1 x 10-5.6 E) [H+] = 1 x 10-2 Question 6 0.5 / 0.5 pts Match the following compounds to the names.
View Related Questions